Abstract
Background:
Anti-CD19 CAR treatment has shown a remarkable response in r/r B-ALL patients, however, a major cause for relapse following CAR administration is the loss of the CD19 antigen. CD22 is a promising alternative target for B-ALL malignancies, although generating an effective CAR to CD22 is challenging due to the size, density and rigidity of this ligand. Furthermore, in the B-ALL setting the CD22 expression levels are known to down regulate in response to selective CAR pressure. This resulted in patients relapsing with lower CD22 density post-treatment (2,839 epitopes/cell) presumably due to the target density falling below the sensitivity threshold for the aCD22 CAR 1. In this study we aim to improve CAR efficacy for the treatment of r/r B-ALL by firstly developing a highly sensitive aCD22 CAR capable of targeting cells that express less than 1000 CD22 molecules per cell. Secondly, we combined this new aCD22 CAR with an aCD19 CAR to generate a dual targeting CD19 and CD22 product via co-transduction for the treatment of paediatric r/r B-ALL as part of an extension cohort of the CARPALL clinical trial (NCT02443831).
Results:
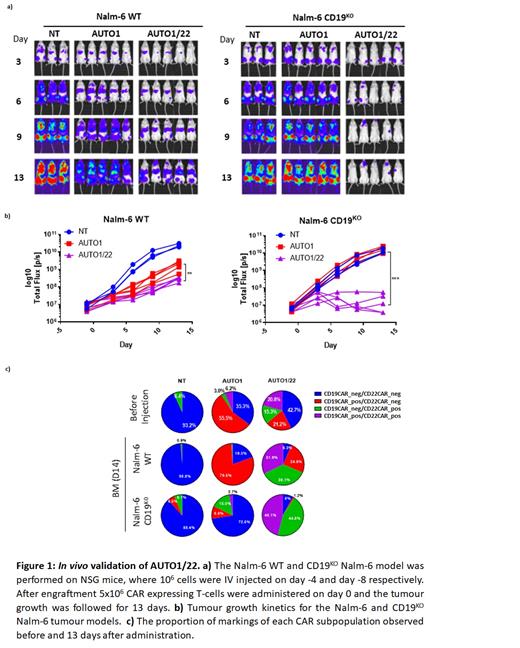
We screened 18 anti-CD22 binders with a range of affinities (1-36.5nM) and binding epitopes in CAR format for high functional sensitivity to low density targets. We filtered binders for their ability to maintain cytotoxicity, proliferation and cytokine release with low expressing CD22 targets. We identified the optimal aCD22 binder (9A8) which when combined in a CAR structure containing a 41BBz endodomain was capable of lysing target cells transduced to express less than 255 CD22 mols/cell while not affecting CD22 negative targets. Similar to the previously tested aCD22 binder M971, our new 9A8 binder also targets the membrane proximal Ig domain of the CD22 molecule. However unlike M971, 9A8 utilises a conventional 16 amino acid linker between the heavy and light chain to form the scFv and has a lower propensity to tonically signal compared to the M971 CAR. To generate a dual CD19 and CD22 targeting product, the 9A8 aCD22 CAR was co-transduced with the aCD19 CAT19 CAR vector (AUTO1) utilised in the CARPALL clinical trial for the treatment of paediatric r/r B-ALL. In a NSG animal model engrafted with WT CD19+/CD22+ Nalm-6 cells, the dual targeting co-transduced T cells (AUTO1/22) were significantly better at supressing tumour growth compared to single targeting AUTO1 CAR T cells (Figure 1). Furthermore, CAR T cells recovered from these animals showed an equal persistence of both aCD19 CAR and aCD22 CAR. In an effort to recapitulate the loss of CD19 target observed in aCD19 CAR relapsed patients, we challenged our transduced T cells in a CD19-knockout Nalm-6 NSG model (Figure 1). In this setting, single transduced AUTO1 CAR T cells were unable to control tumour growth above the non-transduced T cell controls. In contrast, the dual targeting co-transduced AUTO1/22 CAR T cells significantly supressed tumour growth and showed enrichment of CAR T cells expressing the aCD22 9A8 CAR in the bone marrow.
The dual targeting co-transduced AUTO1/22 is currently being evaluated in an extension cohort of the CARPALL clinical trial (NCT02443831) for the treatment of paediatric r/r B-ALL.
Conclusion:
We engineered a highly sensitive aCD22 9A8 CAR which when combined with the aCD19 CAT19 CAR allows for improved in vivo function and the targeting of CD19 negative, CD22 positive cells. The use of these dual targeting T cells may reduce the incidence of CD19 negative relapses in the r/r B-ALL setting.
1. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24(1):20-28.
Kokalaki: Autolus Ltd: Current Employment. Grothier: Autolus Ltd: Ended employment in the past 24 months. Manzoor: Autolus Ltd: Ended employment in the past 24 months. Costu: Autolus Ltd: Ended employment in the past 24 months. Srivastava: Autolus Ltd: Current Employment. Jha: Autolus Ltd: Current Employment. Gealy: Autolus Ltd: Current Employment. Stanczuk: Autolus Ltd: Ended employment in the past 24 months. Robson: Autolus Ltd: Current Employment. Taylor: Autolus Ltd: Current Employment. El-Kholy: Autolus Ltd: Current Employment. Baldan: Autolus Ltd: Current Employment. Righi: Autolus Ltd: Current Employment. Sillibourne: Autolus Ltd: Current Employment. Onuoha: Autolus: Ended employment in the past 24 months. Ghorashian: UCLB: Patents & Royalties; Novartis: Honoraria. Cordoba: Autolus Ltd: Current Employment. Amrolia: ADC Therapeutics: Other: Named inventor on a patent which is being transferred to ADCT.; Autolus: Patents & Royalties. Pule: Autolus Ltd: Current Employment.